Adsorption Databank
-2010-
Moscow State University, Chemistry Dept.
Head supervisor: prof. Tolmachev
A.M.
Executive supervisor: Godovikov I.A.
Executors: Kuznetsova T.A., Kruchenkova N.G., Godovikova
M.I., Borodulina M.V.
http://www.adsorption.ru,
http://www.chem.msu.ru/~Adsorption
This databank is interactive system of browsing and
searching for adsorption data. Currently there are more than 110 isotherms of
individual gas adsorption on macroporous adsorbents,
more than 760 individual systems on microporous
sorbents, more than 80 binary systems from solutions on macroporous
and more than 60 binary systems from solutions on microporous
sorbents, more than 100 binary gas systems on different adsorbents, 10 ternary
systems and data on more than 230 adsorbents produced not only
in Russia but also in other countries. The
bank is constantly expanding.
For
user convenience the results of calculation of experimental data by different
physical-chemical equations are also shown. The constants of corresponding equations
give us important information about adsorption systems [1-3].
The adsorption of individual compounds on macroporous adsorbents. For physical-chemical characterization of
these adsorption systems the equations of BET [4] (1) and Aranovich
[5] (2) were used.:
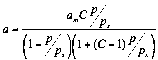 (1)
(1)
The values of am,C and correspondent charts are presented
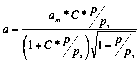 (2)
(2)
The values of am*,C*
and correspondent charts are presented
From here
and further: a – the adsorption
under the pressure P, am, am* - the limit adsorption (the adsorption
capacity), C, C*- the adsorption constants, ![]() - the saturated vapor pressure of the adsorbtive.
- the saturated vapor pressure of the adsorbtive.
The adsorption of individual compounds on microporous adsorbents. We used the equations of Dubinin-Radushkevich (n=2, adsorption on carbons, ps<10bar) or Dubinin-Astachov
(n=3, adsorption on zeolites, ps<10bar)
[6] (3):
![]() (3)
(3)
The values of following constants are presented: the
value of adsorption capacity a0 under the pressure of
saturation Ps and E0 – characteristics adsorption
energy.
With the
values of Рs exceeding 10 bar
all calculations were done only if the volatility data for compounds were
available.
If
the temperature exceeded Tкр (critical
temperature) of the compound we made (if it was possible) the calculation of
equation (3) with using as standard pressures the values of ![]() [7] which were obtained by extrapolation to overcritical temperature
region of the following dependence:.
[7] which were obtained by extrapolation to overcritical temperature
region of the following dependence:.
![]()
(A
and B – constants)
In case
we could obtain the values of vapor densities (![]() ) and liquid densities (
) and liquid densities (![]() ) of the compound under certain temperatures we use also the numeric
solution of the Tolmachev- Aranovich
equation system [8, 9], based on the grid model by Ono-Kondo [10]. In the majority
of cases the real sizes of micropores could contain one
or two layers of adsorbat and thus, the corresponding
equations for mono-(4,6) and bilayer-(5,6) models are [7,9]:
) of the compound under certain temperatures we use also the numeric
solution of the Tolmachev- Aranovich
equation system [8, 9], based on the grid model by Ono-Kondo [10]. In the majority
of cases the real sizes of micropores could contain one
or two layers of adsorbat and thus, the corresponding
equations for mono-(4,6) and bilayer-(5,6) models are [7,9]:
![]() (4)
(4)
![]() (5)
(5)
![]() , n=1 or 2 (6)
, n=1 or 2 (6)
![]() (7)
(7)
From here and further:
![]() - the value of absolute adsorption of the first component,
- the value of absolute adsorption of the first component,
![]() - the adsorption capacity of the first component (mmol*g-1),
Y1, X1 - molar parts of the first component in
equilibrium volume (in this case vacancy solution [8,9]) and equilibrium adsorption
solutions respectively,
- the adsorption capacity of the first component (mmol*g-1),
Y1, X1 - molar parts of the first component in
equilibrium volume (in this case vacancy solution [8,9]) and equilibrium adsorption
solutions respectively, ![]() - coupled
interaction energies of components in adsorption solution, vacancy solution and
with adsorbent respectively.
- coupled
interaction energies of components in adsorption solution, vacancy solution and
with adsorbent respectively.
The
equations (4-7) give us opportunity to describe absolute adsorption isotherms
for both gases and vapors in broad intervals of temperatures and pressures
The values of the following constants are presented: a01, ![]() . If it is necessary
(at T>Tcr)
in field “Notes” we present additionally the values of
. If it is necessary
(at T>Tcr)
in field “Notes” we present additionally the values of ![]() . Below critical
temperatures this parameter practically equals to zero and that’s why it is not
presented.
. Below critical
temperatures this parameter practically equals to zero and that’s why it is not
presented.
Isotherms of exceed adsorption of the components from
binary solutions on macroporous adsorbents were described
by the Aranovich-Tolmachev equation system [1,11] for
monolayer adsorption model. In this case interexchange energies ![]() are practically the same and
the equation system has only 3 parameters:
are practically the same and
the equation system has only 3 parameters:
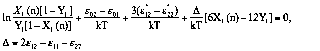 (8)
(8)
![]() (9)
(9)
a1.m- the monolayer adsorption capacity of the first
component, Г1- the value of
exceed adsorption
With the following parameter:
![]() ,
,
![]() - peer
energies of interaction in volume solution
- peer
energies of interaction in volume solution
The values of the following constants are presented: a1m,
B, ![]() (D/kT in forms)
(D/kT in forms)
Isotherms of exceed adsorption of the components from
binary solutions on microporous adsorbents were described
by the 4-parametric Tolmachev-Aranovich equation
system [1,7,12] for bilayer adsorption model:
![]() (10)
(10)
![]() (11)
(11)
![]()
![]() ,
, ![]()
The values of the following constants are presented: a1m,
B, ![]() (D1/kT),
(D1/kT), ![]() (D2/kT)
(D2/kT)
In this
case a1m parameter is formal and it corresponds not to the value of
adsorption capacity [12].
The
values of separation coefficient S for gas mixtures were also presented:
![]() (12)
(12)
Apriori calculations for thernary
systems
Because of very limited number of
experimental data for thernary adsorption systems in
databank we present the results of theoretical calculations for thernary adsorption systems based on experimental data for
corresponding binary adsorptions systems. The possibility of such calculation was
proved by A.M. Tolmachev
and coworkers [1,13]. There it was shown that the calculation of equilibria for adsorption of threecomponent
solutions based on experimental data for correspondent binary solutions could
be done with as high precision as
it is for experimental data receipt
(2-5%).
All mentioned equation constants,
table data for experimental isotherms and isotherm charts were presented in the
databank.
The adsorbent characteristics correspond to the
published in specialized catalogs or technological production data by manufacturing
firms (see corr. references).
References
1. А.М.Толмачев. // Физикохимия
поверхности и защита материалов. 2010. Т. 46. № 3. С. 291. Protection of Metals and Physical Chemistry of
Surfaces.2010. V. 46. №
3. P.242. А.М.Толмачев, И.А.Годовиков // Вестн.Моск. Ун-та.Сер.2.Химия.2001. Т. 42. №4. С. 241.
2. И.А.Годовиков, Т.А.Кузнецова, А.М.Толмачев //
Журн. Физич. химии.2001.Т.75. №11. С. 2030.
3. А.М.Толмачев, О.И.Трубников, И.А.Годовиков, Т.А.Кузнецова // Вестн. Моск. Ун-та. Сер. 2. Химия. 2001. Т.42. №4. С. 247.
4. S. Brunauer, P.H. Emmett, E .Teller. // J. Am. Chem. Soc.
1938. V. 60. P. 309.
5. Г.Л. Аранович. // Журн. физич. химии. 1988. Т. 62. №11. С. 3000.
6. M.M. Dubinin. // Progress in surface and
membrane Sci. New York:Acad.
Press. 1975. V. 9. P. 1. М.М.Дубинин,В.А.Астахов. Изв. АН СССР. Сер.хим. 1971. С. 5,11,17.
7. А.М.Толмачев, М.М.Дубинин, М.Е.Белоусова, А.А.Фомкин. // Изв. АН СССР. Сер. Хим. 1987. C. 19. А.М. Толмачев, Т.А. Кузнецова, И.А. Годовиков. // Физикохимия поверхностных явлений и защита
материалов. 2011. (в печати).
8. A.M.Tolmachev, O.I. Trubnikov. // Carbon. 2002. V. 40 (9). P. 1401.
9. А.М.Толмачев, Ф. Стекли, О.И.Трубников., Т.А.Кузнецова. // Журн. физич. химии. 1999. Т. 73. № 7. С. 1267.
10. С.Оно, С.Кондо.
// Молекулярная теория поверхностного
натяжения в жидкостях. Пер. с англ. М.:МИР. 1963. С. 262.
11. А.М.Толмачев, Е.М.Еремина, О.И.Трубников, Н.А.Окишева // Журн. физич. химии, 1997. Т. 71. № 4. С.682.
12.
А.М.Толмачев, М.И.Годовикова, Т.С.Егорова. // Журн.физич.химии.
2005. Т. 79. № 1. С. 1.
13. А.М Толмачев.// Adsorption, Science and Technology. 1993. V. 10. P. 155. Вестн. Моск. Ун-та. Сер. 2. Химия. 1994. Т. 35. № 2. С. 115.
The
realized databank is not a quiet complete system. The authors will appreciate every
experimental data of different substance
adsorption or of the mixture of substances
adsorption on every absorbents from every source or
person. We accept all the information through our contact mailbox: amtolmach@yandex.ru.
As it was mentioned above currently presented databank
system from one side is the database of adsorption systems and from the other
one is the system of search requests building as standard web-forms. After the start
of corresponding site division user could see the following initial form (Fig.
1):
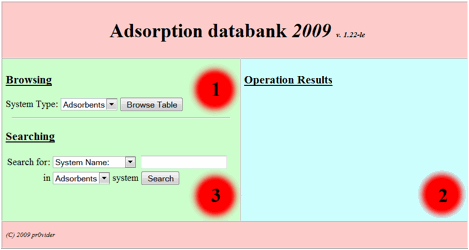
Fig 1.
This basic form divided
on to three main zones: 1. Browsing in the base zone. 2. The result
output zone. 3. Searching in the base zone – the zone for
search request building. So in order to browse the base one should choose interested
division of the base (table to browse) from the following listbox:
Adsorbents (absorbent data)
Binvapor (experimental isotherm data for adsorption of binary
gas (vapor) mixtures on different adsorbents)
Binsmi (experimental
isotherm data for adsorption of binary mixtures from solutions on microporous adsorbents)
Binsma (experimental isotherm data for adsorption of binary
mixtures from solutions on macroporous adsorbents)
Macro (experimental isotherm data for adsorption of
individual compounds on macroporous adsorbents)
Micro (experimental isotherm data for adsorption of
individual compounds on microporous adsorbents)
Ternary (experimental isotherm data for adsorption of triple
(ternary) gas or solution mixtures on different adsorbents)
and press “browse table”
button. After that in the second (output) zone the table output will appear as following: (for
example we choose “Micro” table – the table of experimental isotherm data
for adsorption of individual compounds on microporous
adsorbents and press “browse table” button):
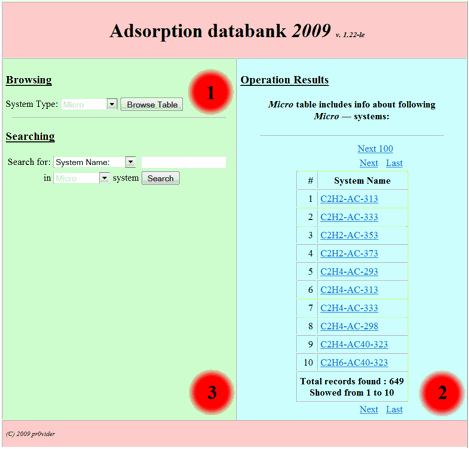
Fig 2
In the second zone
the table per 10 has been appeared. User could move through the table by
pressing management links (Next, Next100, Last, Previous, Previous100, First) or
choose interested system record by clicking on the correspond
system link. After that action all common information for chosen adsorption
system will be presented in separate window as following (Fig 3):
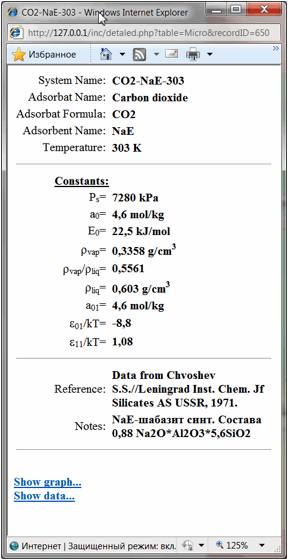
Fig
3.
Here one could see
the basic parameters of chosen adsorption system – i.e. the system name, the
name (names) and the formula (formulas) of adsorbat compounds,
the adsorbent name, the temperature and the basic constants of calculation by
different physical-chemical equations (depends on the system type) – as
it was described above in the first section of this manual:
Adsorbents (adsorbent data): D - adsorbent density, VS - total
pore volume, Vmicro –
micropores volume Vmeso-
mesopores volume Vmacro
– macropores volume, w01,w02 – limit volumes of the sorption
space, E01 - E02- characteristics sorption
energies.
Binsmi (experimental
isotherm data for adsorption of binary mixtures from solutions on microporous adsorbents): a1m,
B, D/kT, D2/kT - parameters of Tolmachev-Aranovich equation for adsorption of mixture components
from solutions on microporous adsorbents (10,11), a01 a02 – adsorption
capacities of components on current adsorbent, obtained from the Dubinin-Radushkevich (Dubinin-Astachov)
equations if any available.
Binsma (experimental
isotherm data for adsorption of binary mixtures from solutions on macroporous adsorbents): a1m,
B, D/kT - parameters of Aranovich-Tolmachev equation for adsorption of mixture components
from solutions on macroporous adsorbents (8,9), a01, a02 – adsorption
capacities of components on current adsorbent, obtained from the BET (Aranovich) equations if any available.
Macro (experimental isotherm data for adsorption of
individual compounds on macroporous adsorbents): ps-
the saturated vapor pressure of the compound under current temperature, am,C – BET equation parameters(1), am*,C*- Aranovich
equation parameters (2).
Micro (experimental isotherm data for adsorption of individual
compounds on microporous adsorbents): ps- the
saturated vapor pressure of the compound under current temperature, rvap, rliq, rvap/rliq – densities
of: vapor, liquid and their ration respectively, a0,E0 – parameters
of Dubinin-Radushkevich (for carbons) or Dubinin-Astachov (for zeolites)
equations, a01, e01/kT, e01/kT – parameters
of Aranovich-Tolmachev equation for adsorption of individual compounds on microporous adsorbents (4-6).
And finally literature
reference and hyperlinks to the data table and data chart are shown. By
pressing them user could open in separate windows the table of experimental
isotherm data (Fig. 4) or isotherm chart (Fig. 5):
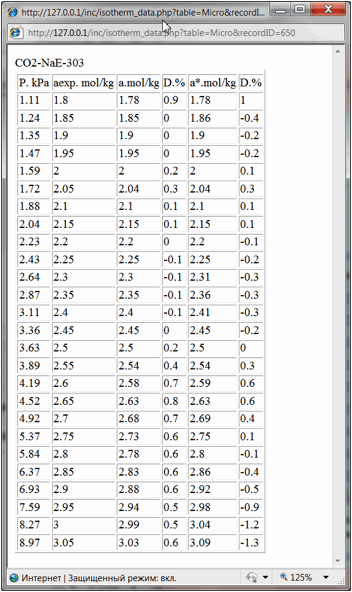
Fig 4.
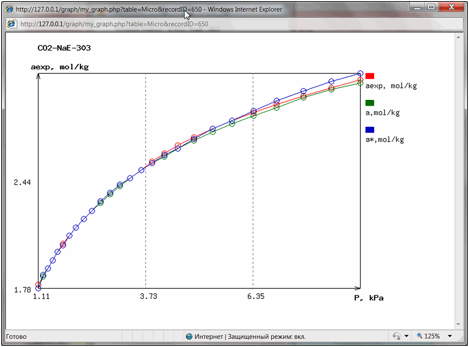
Fig 5.
All presented
data could be easily copied to another application executed or printed in
standard way in operation system.
For
searching in the base one should choose initial search criteria from the listbox available in the zone 3 (System name, Adsorbat name, Adsorbat formula,
Adsorbent name, Temperature), then should enter search variable (or its
fragment) in the corresponding textbox – i.e. what he exactly wants to
find and then should choose the table in which he wants to search (Macro, Micro,
Adsorbents, Binvapor, Binsmi,
Binsma, Ternary). Thus for example the following
combinations will output the following results:
|
Search criteria |
Search variable |
Search table |
Result |
|
Adsorbat name |
Methane |
Macro |
All systems of CH4 adsorption on macroporous adsorbents |
|
Adsorbat formula |
CCl4 |
Binsmi |
All
systems of
binary mixture
adsorption from
solution with
carbon tetrachloride
on all
microporous adsorbents
under all
temperatures available |
|
Adsorbent name |
SKT |
Micro |
All systems of adsorption of all individual compounds
available on microporous adsorbents which contain «SKT»
in its name under all temperatures available |
|
Temperature |
303 |
Micro |
All systems of adsorption of all substances available
on all microporous adsorbents under 303K |
|
System Name |
303 |
Micro |
All systems of adsorption of all substances available
on all microporous adsorbents under 303K |
|
System Name |
СH4-SKT |
Micro |
All systems of CH4 adsorption on adsorbents
which contain in its name «SKT» under all temperatures available. |
|
System Name |
CCl4-C6H6 |
Binsmi |
All adsorption systems from the mixtures of carbon
tetrachloride with benzene on all adsorbents available under all
temperatures. |
|
System Name |
CCl4-C6H6-AC |
Binsmi |
All adsorption systems from the mixtures of carbon
tetrachloride with benzene on AC adsorbent under all temperatures. |
|
System Name |
C3H8 |
Micro |
All systems of adsorption of C3H8 on all microporous adsorbents available under all temperatures (Fig.
6) |
After pressing “Search”
button the search result will be output as table in the second zone. It consists
of the names of adsorption systems which satisfy search
conditions entered by user as following (Fig 6):
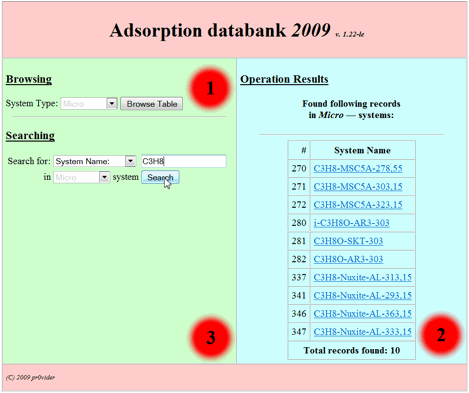
Fig 6
After that by
clicking on the interested system link one could have isotherm common data, isotherm
table data and isotherm chart from the obtained table as it was described above.
With all suggestions, notes and questions one could
send mail to:
amtolmach@yandex.ru, pr0vider@yandex.ru,
tchess54@mail.ru.